Abstract
Sickle cell anemia (SCA) is an inherited red blood cell disorder that leads to kidney disease in up to two-thirds of adults. Ultrasound is a non-invasive modality that can identify pathologic changes in the kidney. Previous studies have demonstrated that SCA patients have larger and more echogenic kidneys compared to non-SCA patients, but the association of abnormal kidney ultrasound findings with kidney function or with the APOL1 G1/G2 kidney risk variants in SCA is not clear.
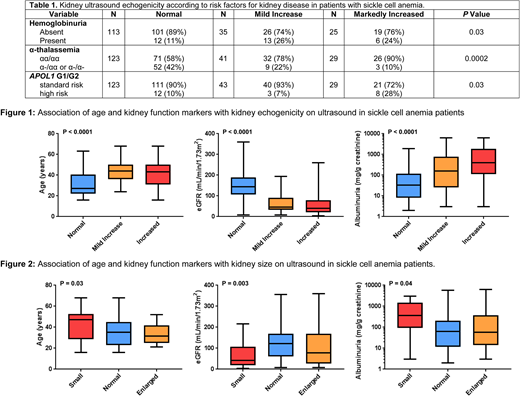
We conducted a retrospective analysis to evaluate the association of kidney ultrasound findings with markers of kidney function and the APOL1 G1/G2 risk variants at our institution. Clinical and laboratory variables were collected closest to the time of the ultrasound. Ultrasounds were independently reviewed by radiologists who were blinded to the measures of kidney function and APOL1 G1/G2 risk status. APOL1 G1 and G2 and α-thalassemia status were determined by PCR. Homozygous or compound heterozygous inheritance of the G1 and/or G2 risk variants was defined as high risk. Echogenicity was classified as normal if the renal cortex was less echogenic than the adjacent liver or spleen, mild if the renal cortex was the same or mildly more echogenic than the adjacent liver or spleen, and markedly increased if the renal cortex echogenicity was obliterating the renal sinus. Normal kidney size ranges were defined as 10-14cm in men and 9-13cm in women. Hemoglobinuria was defined as urinalysis positive for blood by dipstick with < 2 red blood cells per high power field. Comparisons of linear and categorical variables by degree of echogenicity or kidney size were performed using the linear trend test or Cochran's test of linear trend, respectively.
Between 2/2002 and 2/2018, 199 SCA patients had an abdominal or retroperitoneal ultrasound performed with assessment of the kidneys. The median age of the cohort was 35 years (interquartile range [IQR], 24-46 years) and 37% were male. Echogenicity of the kidneys was normal in 125 (63%), mildly increased in 43 (22%), and markedly increased in 31 (16%) SCA patients. Incremental kidney echogenicity was associated with older age, increased albuminuria, and lower estimated glomerular filtration rate (eGFR) (P<0.0001; Figure 1). Kidney echogenicity was greater in SCA patients with hemoglobinuria (P=0.03). Co-inheritance of α-thalassemia was associated with reduced kidney echogenicity (P=0.0002) and high risk APOL1 G1/G2 status was associated with increased kidney echogenicity (P=0.03). On logistic regression analysis, abnormal kidney echogenicity was independently associated with older age (10-year increase OR 2.6, P<0.0001), α-thalassemia status (OR 0.3, P<0.0001), and APOL1 G1/G2 risk status (OR 4.9, P=0.007).
Kidney size measurements were categorized as small in 20 (10%), normal size in 157 (79%), and large in 22 (11%) of SCA patients. Smaller kidneys were associated with older age (P=0.03), increased albuminuria (P=0.04) and a lower eGFR (P=0.003) (Figure 2). We did not observe significant associations between kidney size and hemoglobinuria, α-thalassemia status, or APOL1 G1/G2 risk variant status.
In conclusion, we demonstrate that in SCA patients, increased kidney echogenicity and smaller kidney size are associated with older age, reduced eGFR, and worsening albuminuria. Furthermore, absence of α-thalassemia, and presence of the APOL1 G1/G2 kidney risk variants are associated with the degree of kidney echogenicity. Future studies evaluating the utility of kidney ultrasounds as a clinical tool to monitor kidney disease and its progression in SCA are warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal